

ĭid you know the Henderson-Hasselbalch equation is also crucial in determining the isoelectric point of a substance. The Henderson-Hasselbalch equation is derived from the definition of the acid dissociation constant: pH pK a + log 10 (A / HA). The p of the K constant (pK), as mentioned above, is equal to the pH of a solution that contains precisely the same amount of dissociated and undissociated acid, =. This equation is also known as Henderson-Hasselbalch equation. The equilibrium constant (K) for this equation looks like follows: This calculator is valid for a buffer of a weak acid and its conjugate base of the same. That's how we can create a conjugate pair of acids & bases. However, as it uses only the Henderson-Hasselbalch equation and doesnt account for other components of the. Du har å gjøre med en bufferløsning som inneholder pyridin. The base is a proton acceptor (is willing to accept a particle of hydrogen: ⁻).Įvery acid dissociates into anions and cations when dissolved in water, HA ⇌ H⁺ + A⁻. This is useful for creating fine pH gradients. For å kunne bruke Henderson-Hasselbalch-ligningen, som for en buffer som inneholder en svak base og dens konjugat, ser ut som denne fargen (blå) ( bar (ul (farge (hvit) (a / a) 'pOH' pKb + log / svak base) farge (hvit) (a / a) ))) du må bestem.The acid can be described as a proton donor (contains a particle of hydrogen: H) and.The HH equation is used for calculating the pH and concentration of a buffer - a solution that consists of a strong acid and a weak, conjugate base, or a strong base and a weak, conjugate acid. To describe how a buffer solution (either acidic or basic) can resist.
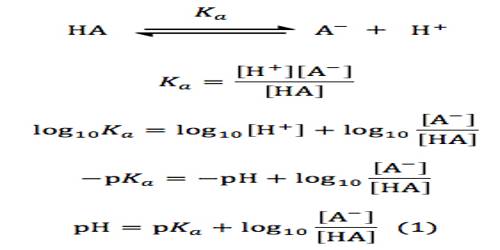
It's time for a small bit of math revision: p is a symbol for a negative logarithm, with a base of 10 (for example, pH = -log₁₀(H)). The principal objectives of the Henderson Hasselbalch equation include the following: To calculate the pH, pOH, H3O+ tot, OH- tot, H3O+ water, and OH- water in a solution containing a strong acid (base) given the initial concentration of the acid (base). The Henderson-Hasselbalch equation for pH looks like this:


 0 kommentar(er)
0 kommentar(er)
